Collaborative platform for PCD diagnosis
The "Work Package 7 - Improving PCD Diagnosis” has created a slack workspace to provide a collaborative platform about PCD diagnosis. In specific channels, BEAT-PCD members will have the opportunity to ask questions about protocols and methods and to share difficult cases (TEM, HSVM and IF).
Slack allows the sharing of images, videos or PDF files on the platform. The platform will be monitored and moderated at least twice a week by WP7 members providing answers or resources. We are asking members to keep opinion pieces to the minimum and we encourage the exchange of only evidence-based information.
If you would like to join, please contact Dr Mathieu Bottier to receive an invite.
Standardised PCD-specific clinical data collection
FOLLOW-PCD is a standardised disease-specific instrument for recording information collected during baseline and routine follow-up visits of patients with PCD (Goutaki et al. ERJ Open Research 2020 6: 00237-2019). It was developed by an interdisciplinary working group of researchers, clinicians and other healthcare professionals working with PCD, in the framework of the BEAT-PCD COST Action. The tool has a module structure including clinical modules to be completed by different healthcare professionals and a patient questionnaire to be completed by patients or caregivers.
Currently FOLLOW-PCD is piloted in PCD outpatients clinics to test the instrument in real-life conditions and identify items that need to be added or eliminated or if variables must be recoded or further clarified. Based on the results from the piloting phase, we will develop a refined form (FOLLOW-PCD version 2.0). The patient questionnaire has 3 age-related versions and is already available in English, German, Greek, French, Norwegian, Dutch/Flemish, Turkish, Spanish, Danish and Ukranian.
Centres that wish to pilot the form or translate the patient questionnaire should contact Myrofora Goutaki for more information.
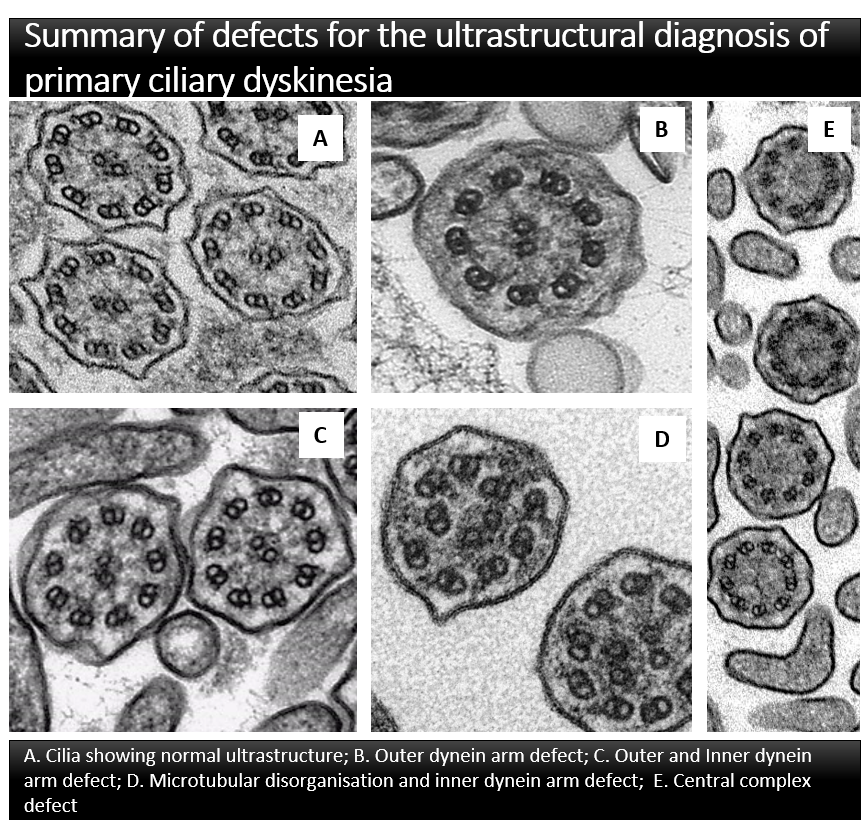
A consensus guideline was developed by PCD electron microscopy experts representing 18 centres in 14 countries to provide an internationally agreed ultrastructural classification for the diagnosis of PCD. The final guideline: Provides agreed terminology and a definition of class 1 defects which are diagnostic for PCD; Identifies class 2 defects which can indicate a diagnosis of PCD in combination with other supporting evidence; Describes features which should be included in a ciliary ultrastructure report to assist multidisciplinary diagnosis of PCD; Defines adequacy of a diagnostic sample.